- Since June 2008, independent Research Cell has been working with the aim of providing thrust to the research activities to be carried out at Sumandeep Vidyapeeth.
- The Research Cell works with a specific perspective of encouraging the faculty of the University in conducting research work – viz. writing basic research protocols, orientation to the regulatory requirements, managing all the research carried out within the ICH-GCP frame and looking after the Ethical requirements. The Research Cell also has a fully functional Institutional Ethics Committee and Animal Ethics Committee as per the requirement of regulatory bodies. There is individual Human Research Review Panel (HRRP) of the individual institute. All the institutes HRRP are following the general guideline so research cell and SOP of SVIEC which is also based on the ICMR, Schedule-Y and ICH – GCP guidelines.
- SVDU has received DSIR recognition (SIRO certificate) in April 2016 and eligible for receiving external research grant from the national funding agencies. We at Research Cell encouraging our faculty for submission of high end research projects in collaboration with other eminent institutions in order to develop new processes and products from the bench to the bedside in a one go in order to improve quality and convenience of users. We also have collaboration with other national & International Universities, NGOs, Government agencies and industries, for execution of high end projects of societal interest.
| Sr. No | Collaborative Institution | Purpose of Collaboration |
|---|---|---|
| 1 | University of Hull | Collaboration for educational acitivities, to promote the sharing of knowledge, exchange of information & expericences for staff &students |
| 2 | Avalon University School of Medicine | Collaboration for research, faculty and student exchange, PhD collaborations and develoment of courses on skill development, allied health programs |
| 3 | The Orenburg State Medical University | Collaboration for Cooperation in education, science & medical activities, in the sphere of experimantal research work,to support each other by providing research & study material |
| 4 | University of Rochester | Collaboration for Joint educational & research acitivties, exchange of scholars, undergraduate & postgraduate students, scholary information exchange, No financial support from both side |
| 5 | MINDS, USA | Collaboration for providing mental health education workshops & screening for those in need to psychiatric care, collabrate on research projects relevant to psychiatry,neuroscience,mntal health |
| 6 | Mount Sinai Services of the Icahn School of Medicine | Collaboration for Psychiatry Trainig Program |
| 7 | Center of Clinical Neuroscience, University medical Center Hamburg Eppendorf | Collaboration for Joint educational & research activities, Exchange of faculty, students and scholary information,joint PHD program and twinning program |
| 8 | Egyptian Association for International Medical Studies – Cairo Univeristy, Egypt | Collaboration for For bilateral cooperation for development & dissemination of medical knowledge |
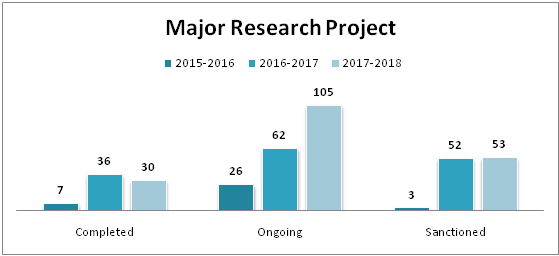
| Major Research Projects | |||
|---|---|---|---|
| Completed | Ongoing | Sanctioned | |
| 2015-2016 | 7 | 26 | 3 |
| 2016-2017 | 36 | 62 | 52 |
| 2017-2018 | 30 | 105 | 53 |
| Minor Research Projects | |||
|---|---|---|---|
| Completed | Ongoing | Sanctioned | |
| 2015-2016 | 356 | 466 | 388 |
| 2016-2017 | 287 | 538 | 382 |
| 2017-2018 | 216 | 425 | 219 |

| Sr.No | Name of Investigators | Title of Project | Approval Date | Amount sanctioned (Rs.) | Major/ Minor |
|---|---|---|---|---|---|
| 1 | Ms. Ria Anjaria Ms. Megha Patel |
To evaluate the efficacy of care & care (a product developed at SRISTI) for cracks in heels. | 28-Mar-17 | 100000 | Major |
| 2 | Mr. Aditya Mundra Mr. Saurabhv Mamtani Ms. Dimple Jain Guide: Dr. Vikas Chandrakar |
To evaluate the efficacy of pain relief a product developed at SRISTI for muscular pain. | 3-Feb-17 | 100000 | Major |
| 3 | PIs: Mr. Alok Rai Ms. Mahek Mistry Mr. Kunal Singh Guide: Dr. Vikas Chandrakar |
To evaluate the efficacy of mosquito a product developed at SRISTI for repelling Mosquito. | 3-Feb-17 | 100000 | Major |
| 4 | Dr. Girish Sailor | Phyto-pharmacological screening and formulation development of some medicinal mushroom | 18-May-15 | 165000 | Major |
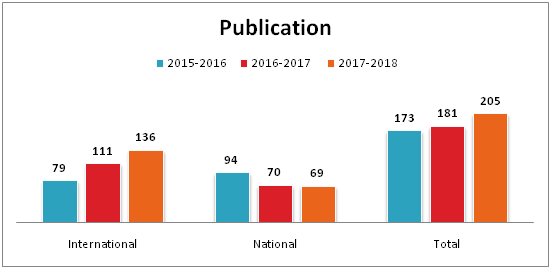
| Number of Publications | |||
|---|---|---|---|
| Institute | 2015-2016 | 2016-2017 | 2017-2018 |
| Department of Pharmacy | 19 | 19 | 11 |
| Sumandeep Nursing College | 25 | 7 | 21 |
| Department of Management | 3 | 5 | 5 |
| College of Physiotherapy | 0 | 1 | 8 |
| K.M.Shah Dental College & Hospital | 42 | 61 | 48 |
| Smt. B.K.Shah Medical Institute & Research Centre | 84 | 88 | 112 |
| Total | 173 | 181 | 205 |
| Year | International | National | Total |
|---|---|---|---|
| 2015-2016 | 79 | 94 | 173 |
| 2016-2017 | 111 | 70 | 181 |
| 2017-2018 | 136 | 69 | 205 |
CLINICAL TRIALS
|
Sr. No |
Name of Principal Investigator |
Project Title |
Status |
Year |
|---|---|---|---|---|
|
1. |
A Randomized, Double Blind, Double Dummy, Comparative, Prospective, Multicenter, Parallel Study to assess Efficacy, Safety and Tolerability of Fixed Dose Combination of Teneligliptin and Metformin Tablet Compared with Metformin monotherapy in Adult Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin Alone |
Completed |
2015-2016 |
|
|
2. |
Dr. Punit Singh |
A multicentre, double blind, active controlled, parallel group, two arm, bioequivalence study with clinical endpoint comparing brinzolamide 1% ophthalmic suspension (manufactured by Indoco remedies ltd., for Watson pharmapvt ltd.), to brinzolamide (azopt®) 1% ophthalmic suspension of Alcon laboratories inc., in the treatment of chronic open angle glaucoma or ocular hypertension in both eyes |
Completed |
2015-2016 |
|
3. |
Dr. Dulari Gandhi |
A Phase 4/3, Open-Label, Single-Arm, Multicenter Study to Describe the Safety and Immunogenicity of 13-Valent Pneumococcal Conjugate Vaccine in Adults 50 to 65 Years of Age and in Children 6 to 17 Years of Age in India |
Completed |
2015-2016 |
|
4. |
Dr. Dulari Gandhi |
A Bridging study to evaluate Immunogenicity and Safety of a Pentavalent vaccine (DTwP-HepB-PRP-T) Shan5 (with Shantha pertussis) as compared to licensed vaccine, Shan5 (with imported pertussis) when administered as three dose primary series at 6-8, 10-12, and 14-16 Weeks of Age in Healthy Indian Infants |
Completed |
2015-2016 |
|
5. |
Dr. Kishan Ninama |
A randomized, double blind, placebo controlled, three arm, parallel group, multi centric, clinical study to evaluate the therapeutic bio-equivalence of two tacrolimus 0.03% topical ointment formulation in adult patients with moderate to severe atopic dermatitis |
Completed |
2017-2018 |
|
6. |
Dr. Rashmi Mahajan |
A Multi-Centre, Randomized, Double Blind, Parallel-Group, Comparative Clinical Trial to evaluate the Safety and Clinical Equivalence of Generic Clotrimazole Troche/Lozenges USP, 10mg (Unique Pharmaceutical Laboratories, India) to Clotrimazole Troche /Lozenges ® 10mg (Roxane Laboratories Inc., USA) in subjects with Oropharyngeal Candidiasis |
Ongoing |
2017-2018 |
|
7 |
Dr Kishan Ninama |
A Phase III, multicentre, randomized, observer blind, parallel group, three arms, controlled clinical trial to evaluate the efficacy and safety of topically applied Calcipotriol/AKVANO 50 μg/g cutaneous solution against Calcipotriol Ointment 50 micrograms/g, Sandoz and placebo in patients with mild to moderate plaque psoriasis |
Ongoing |
2017-2018 |
|
8 |
Dr Kishan Ninama |
A Randomised, Double-blind, Multicentre, Parallel-group, Active & Placebo Controlled, Three Arm Clinical Study to Compare the Efficacy and Safety of Clindamycin Phosphate 10 mg/g + Benzoyl Peroxide 50 mg/g Gel (Morningside Healthcare Ltd, UK) versus DUAC® Once Daily 10 mg/g + 50 mg/g Gel (GlaxoSmithKline UK Limited) in Subjects with Acne Vulgaris |
Ongoing |
2018-2019 |
|
9 |
Dr Arti Shah |
A Phase IV, Prospective, Open Label, Non-Comparative, Multicenter Study With 24 Weeks of Treatment Period To Assess Safety, Tolerability And Efficacy of Pirfenidone 200mg Oral Tablets In Patients With Idiopathic Pulmonary Fibrosis (IPF) |
Ongoing |
2018-2019 |
|
10 |
Dr Chaitri Shah |
Randomized, Double blind, Two Arm, Comparative Controlled, Prospective Clinical Trial of Mycobacterium w in Combination with Standard Therapy versus Standard Therapy Alone in Sepsis due to Gram Negative Infection |
Ongoing |
2017-2018 |
|
11 |
Dr R N Kothari |
A Phase 3, Multi-center, Randomized, Double-Masked Study to Evaluate the Clinical Efficacy and Safety of SHP640 (PVP-Iodine 0.6% and Dexamethasone 0.1%) Ophthalmic Suspension Compared to PVP-Iodine and Placebo in the Treatment of Adenoviral Conjunctivitis |
Ongoing |
2017-2018 |
PRECLINICAL TRIALS
We have an Animal House approved by national Ethics Committee. We are performing all preclinical experiments under the controlled and supervision of experiment on animal’s (CPCSEA). We offer our expertise to all pharmaceutical industries for the preclinical testing for their products and new drug development. We also undertake the animal experimentation for testing toxicity and the pharmacological effect of herbal drugs as part of new drug development.
Contact:
- Dr. A. K. Seth (09824466105)
- Dr. Niraj Pandit (09825371135)
- Email: director.research@sumandeepvidyapeethdu.edu.in
- Non Communicable disease associated with CVS ,Oncology, respiratory & disorder, and ophthalmology
- Communicable disease – diarrhea , viral infection , and malaria
- Proteomics and Genomics in Potentially Malignant Lesions in Oral cavity (Oral leukoplakia, Oral Sub mucous fibrosis, Oral Lichen Planus) and Oral Malignancy
- Life-Style disorder and metabolic disease
- Structured physiotherapy and geriatric patients
- The role of vitamin D and Thiamine in different types of Headaches
- Identification and molecular characterization of active principles from herbal sources for the treatment of metabolic diseases
- Drug withdrawal and Postoperative EEG in epilepsy surgery
- Stem Cell and tissue engineering
- Salivary biomarkers for assessment of cervical lymph node metastasis
- Drug withdrawal and Postoperative EEG in epilepsy surgery
- Traditional medicines and drug delivery
- Biomaterials and medical devices
- Sickle Cell and its trait analysis in Gujarat region